Leak testing requirements
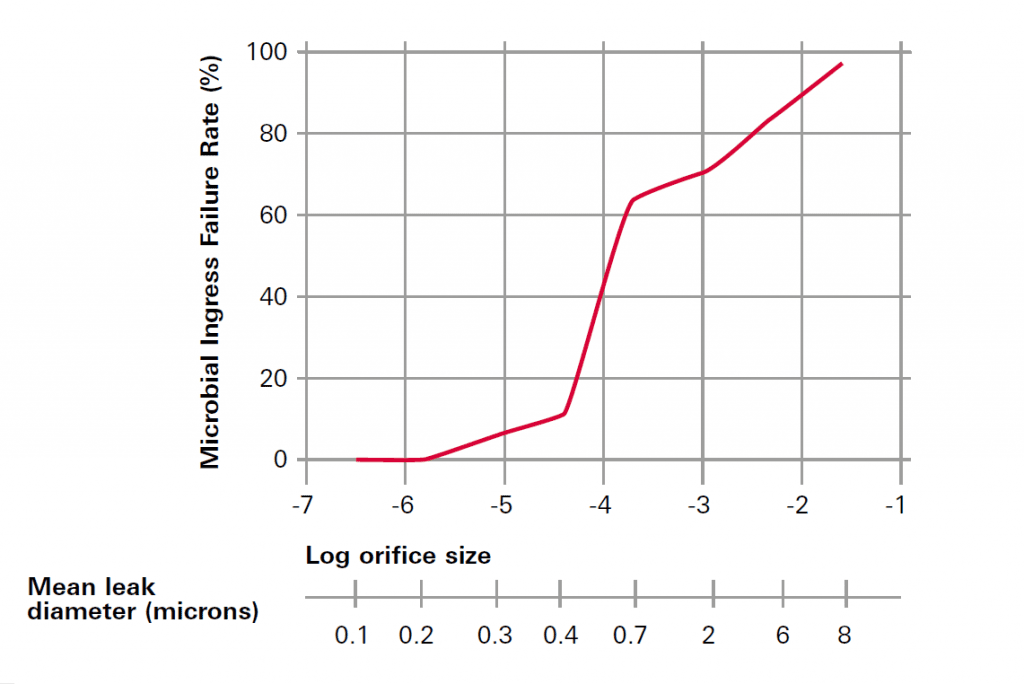
While in the early development stage of packaging, the supplier is obliged to ensure that the packaging is by design capable of ensuring sterility. Therefore, the packaging must to be tested for defects in the range of 0.2 µm, respectively 6 * 10-6 mbar*l/s („maximum allowable leak level“; MALL). Based on a study from Kirsh, this is the defect size where the microbial ingress failure rate is 0 %. Integrity tests are mainly performed in the range of 2 to 20 µm defect size. The main reason for this is the feasibility of the available methods to detect smaller defects in a reasonable test time. When dealing with 100 % inspection of the production line that operates at speeds for 120 to 600 parts per minute, the allowed defect size is sometimes even increased to a significantly higher level. To compensate on the risk-based approach, additional off-line sample testing is performed to a tighter specification in the range of 1 to 10 µm. This also applies to stability testing which is performed in laboratory tests. Here again the sensitivity is more important than the test time.
As there is no one-size fits all solution, Pfeiffer Vacuum offers a unique portfolio of equipment dedicated to the pharmaceutical industry. Using three different technologies for Container Closure Integrity Testing (CCIT). The patented and USP <1207> recognized Mass Extraction Technology and the patented O.E.S. (Optical Emission Spectroscopy) instrument used in the AMI system are used for reliable laboratory testing as well as in process control during production testing. Moreover, Pfeiffer Vacuum delivers a unique turnkey solution for laboratory testing down to the MALL based on helium mass spectrometry. [more]
In addition Pfeiffer Vacuum established a leak testing service to perform feasibility tests for our customers. In a feasibility study for container closure integrity testing, we gain an understanding of your packaging. With our three CCIT-technologies we will confirm achievable detection limit and cycle time on your packaging system. [more]
Container Closure Integrity Testing (CCIT)
The quality and effectiveness of drugs depends significantly on their proper packaging: Sterile products and moisture/oxygen sensitive drugs require excellent barrier during the shelf life of the product (up to a couple of years) to protect them from biological contamination, water and oxygen ingress. Otherwise, serious consequences might occur.
Oxygen / Moisture / Biological Contamination

The high risk in regard to pharmaceutical Container Closure Integrity Testing (CCIT) leads to a strictly regulated environment. What the official regulations often do not describe in detail is how the CCI testing should be performed. They typically only stipulate to use appropriate methods and procedures. The United States Pharmacopeia, the government body in-charge of standards and guidelines for the pharmaceutical industry – which typically are internationally accepted – dealt with this issue and in 2016 presented a new guideline: the USP <1207>. This guideline focuses on sterile and critical pharmaceutical products (e. g. vials and syringes).
All leak testing methods and devices provided by Pfeiffer Vacuum fulfill the requirements mentioned in the USP <1207>. All these methods can be described as:
- Deterministic
- Non-destructive
- Operator independent
- Easy to use
- Sensitive
Moreover, Mass Extraction and Helium Mass Spectrometry are explicitly mentioned in the USP, while the O.E.S. can be evaluated as emerging technology that fulfills these requirements. Pfeiffer Vacuum will be your partner in understanding your CCIT challenge and in finding out which technology will be the best for you.







